Clinical Research & Trials
Clinical Research & Trials
Research is how we move from uncertainty to answers. It’s how we begin to imagine better days with fewer seizures, improved movement, clearer communication, and new options for treatment.
While there are no clinical trials currently underway, researchers across the globe are working on targeted therapies with approaches rooted in the science of potassium channels and powered by KCNA2 patient data. Your participation, your story, and your support make this work possible.


KCNA2 International Registry / Natural History Study
What is it?
The KCNA2 Registry is an international database of genetic, medical, and developmental data from KCNA2 patients worldwide. It is a series of surveys completed by patient families, and then analyzed by research and medical experts. They will publish a Natural History Study using the data that will help us better understand the condition, improve support for families, and accelerate the development of treatments/cures.
Why join?
Your story matters! KCNA2 patients are unique and very small in number so every one of you improves our understanding and course of the disease. This leads to better treatment and care for our loved ones.
Goals of the registry:
- Track how KCNA2 presents and progresses over time (Natural History Study)
- Identify patterns in symptoms and responses to treatment
- Discover more targeted and effective therapies
- Connect the KCNA2 community of families, clinicians, and researchers


What’s involved and how do I start?
The registry takes about 30-45 minutes to complete. You will fill out the surveys online and will receive a unique URL so that you can stop and return at any time. Once a year we will ask you about any changes in the patient’s disease presentation and impact on your life. Join now!


Is my information kept private and secure?
Yes! The registry is hosted on a secure platform that complies with medical confidentiality and data protection regulations. You can read more about this in the consent forms.


Recommended documents to collect before starting:
- Genetic testing report
- Birth report of weight, length, head circumference
- Brain MRI reports
- EEG reports (first EEG, most recent, and any other important ones)
- Medication list (compound names used)
- List of diagnoses and health conditions
Therapeutic Pathways Under Investigation
Even without a current trial underway, KCNA2 researchers are advancing treatment possibilities through multiple promising approaches.
Antisense Oligonucleotides (ASOs)
- Goal: Use ASOs to silence dominant negative variants of KCNA2
- Status: In preclinical development using iPSC lines and animal models
- Reference: Huang et al., 2024 (Singapore-based study): Targeting heterozygous dominant negative variant of KCNA2 using Gapmer ASO.
Small Molecule Compounds
- Location: Canada (University of Toronto), Italy (University of Bari)
- Goal: Create compounds that rescue channel function in patient-derived cells
- Status: In vitro screening underway with expansion planned
- Reference: Preclinical data from University of Toronto and University of Bari research teams. Results are not yet published.
Drug Repurposing: 4-Aminopyridine (4-AP)
- Location: Germany
- Goal: Reuse existing medication (approved for MS) to support seizure reduction in GOF patients
- Study: Read the 2021 study on PubMed
- Status: Ongoing observational use in some clinical cases
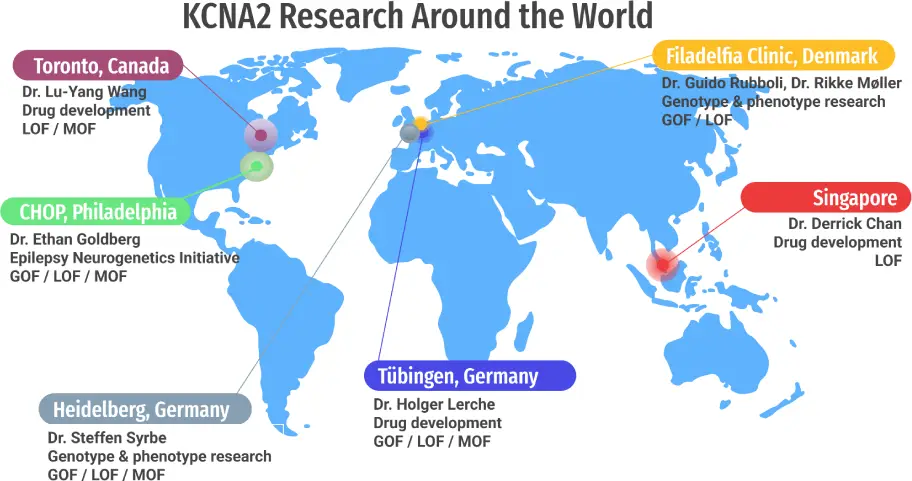
Where KCNA2 Research Is Happening
We’re not just tracking KCNA2 research, we’re actively helping it grow. Our Board is in direct, ongoing communication with researchers across these global sites. In fact, we regularly host Zoom calls (often weekly) to ask questions, share insights, and encourage researchers to connect with one another.
In some cases, we’ve helped scientists find each other for the first time, opening doors for new conversations and new collaborations.
- Germany — University of Tübingen, University of Heidelberg
- Canada — University of Toronto
- Italy — University of Bari
- Denmark — Filadelfia Epilepsy Hospital
- China — Wuxi Biologics
- United States — CHOP (historical), Mayo Clinic (ongoing case studies)
- Ireland — iPSC line developed. No current projects.
Clinical Studies & Trials (Past and Ongoing)
Two GOF patients were treated with Ampyra (4-AP) to assess its impact on seizure activity and motor function. Both showed improvements in seizure frequency, clarity of thought, and balance.
United States: Mayo Clinic Case Reports
Two GOF patients were treated with Ampyra (4-AP) to assess its impact on seizure activity and motor function. Both showed improvements in seizure frequency, clarity of thought, and balance.
Europe: 23-Patient Clinical Study (led by Denmark)

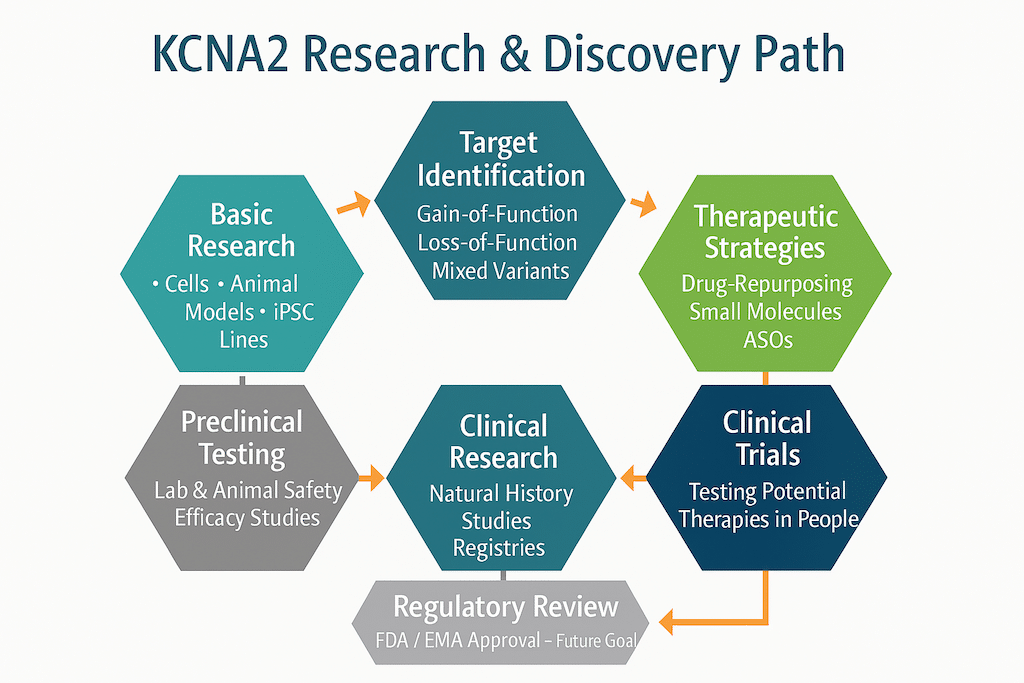
Understanding The Road To Treatment
From identifying a variant to launching a trial, each step takes time, data, and dedicated collaboration. With your help, we’re building the foundation.
Your donation helps fund breakthrough KCNA2 research. Support the path to treatments today.
KCNA2 Epilepsy Family Contact Form
This is our KCNA2 family contact list separate from the KCNA2 International Registry.
Join our family list to stay connected with researchers and KCNA2 families. This list allows us to better coordinate communications, match families with research or surveys, and send news and updates relevant to your variant or region.

What it is:
A secure database of KCNA2 patients and families.

Why it matters:
We will keep you informed about research and clinical trial opportunities, share educational resources, and help families connect with each other.

Privacy:
Your identifying information will never be shared publicly, only used for communication purposes.

How to join:
Still searching for something?
Let’s keep going. We see you. We’re with you. And we’re not giving up.
KCNA2 Epilepsy Foundation | A 501(c)(3) nonprofit organization